
The Lasting Effects of Psychological Trauma on Memory and the Hippocampus
J. Douglas Bremner, M.D.
Departments of Diagnostic Radiology and Psychiatry,
Yale University School of Medicine, Yale Psychiatric Institute,
and National Center for PTSD-VA Connecticut Healthcare System
Dr. Bremner reports no commercial conflict of interest.
This research reviewed in this paper was supported by a NIH-sponsored General Clinical Research Center (GCRC) Clinical Associate Physician (CAP) Award and a VA Research Career Development Award to Dr. Bremner, and the National Center for PTSD Grant.
Educational Objectives
Upon completion of this Cyberounds®, the participant should be able to:
- Discuss how extreme stressors, including childhood abuse and combat, can have lasting effects on hippocampal-based verbal declarative memory and what the relevance of this to education, public policy, rehabilitation and psychiatric treatment
- Describe research findings showing reduction in volume of the hippocampus in posttraumatic stress disorder related to abuse or combat andtheoretical explanations for findings
- Describe how dysfunction of medial prefrontal cortex may contribute to symptoms of stress-related disorders like PTSD.
The invisible epidemic
The invisible epidemic of childhood abuse and other psychological traumas and stressors represents a major public health problem in our society today. Childhood sexual abuse alone affects 16% of women (about 40 million) in the U.S.A. (including rape, attempted rape, or molestation) at some time before their 18th birthday.1
Childhood abuse is the most common cause of posttraumatic stress disorder (PTSD) in women, which affects 8% of the population at some time in their lives,2 although there are a range of other types of psychological trauma that can also lead to symptoms of chronic PTSD, including car accidents, combat, rape and assault. Some of the symptoms of PTSD, which include intrusive memories, nightmares, flashbacks, increased startle and vigilance, social impairment and problems with memory and concentration, may be related to the effects of extreme stress on the brain.3,4
Individuals with a history of exposure to childhood abuse or combat had a reduction in volume of a brain area involved in learning and memory called the hippocampus, which is felt to be related to stress, with associated deficits in hippocampal-based learning and memory.5 Children under stress develop impairments in academic achievement that are specifically related to the development of PTSD. Other symptoms, including fragmentation of memory, intrusive memories, flashbacks, dissociation and pathological emotions, may also be related to hippocampal dysfunction6 and may explain delayed recall of childhood abuse.7 The hippocampus has important links to the medial prefrontal cortex, another brain area that mediates emotion and the stress response, dysfunction of which has also been implicated in PTSD.
A disease of memory
Alterations in memory form an important part of the clinical presentation of patients with PTSD. PTSD patients report deficits in declarative memory (remembering facts or lists, as reviewed below), fragmentation of memories (both autobiographical and trauma-related) and dissociative amnesia (gaps in memory that can occur for minutes to days and are not due to ordinary forgetting).
Psychiatric Symptoms Associated with Childhood Abuse
PTSD
- Nightmares
- Flashbacks
- Memory & Concentration problems
- Hyperarousal
- Hypervigilance
- Intrusive memories
- Avoidance
- Startle reponses
- Feeling worse with traumatic reminders
Dissociative
- Out of body experiences
- Derealization
- Amnesia
- Fragmented sense of self & identity
Anxiety
- Panic attacks
- Claustrophobia
Substance Abuse
- Alcoholism
- Opiate addiction
Many abuse victims claim to remember only certain aspects of the abuse event. For instance, a patient who was locked in the closet had an isolated memory of the smell of old clothes and the sound of a clock ticking. Later, she connected that with feelings of intense fear and, then, the entire circumstances relating to the abusive events. PTSD is also associated with alterations in non-declarative memory (i.e., types of memory that cannot be willfully brought up into the conscious mind, including motor memory, such as how to ride a bicycle). These types of non-declarative memories include conditioned responses and abnormal reliving of traumatic memories following exposure to situationally appropriate cues. Many of these memory disturbances may be related to dysfunction of the hippocampus and related brain areas such as medial prefrontal cortex.
Effects of psychological trauma on the hippocampus and memory
Childhood abuse and other extreme stressors can have lasting effects on brain areas involved in memory and emotion. The hippocampus is a brain area involved in learning and memory that is particularly sensitive to stress.8,9 As reviewed in greater detail by Bruce McEwen in other Cyberounds high levels of glucocorticoids (cortisol in the human) released during stress were associated with damage to neurons in the CA3 region of the hippocampus, and a loss of neurons and dendritic branching.10,11,12 Glucocorticoids disrupt cellular metabolism and increase the vulnerability of hippocampal neurons to excitatory amino acids like glutamate.13 Other neurochemical systems interact with glucocorticoids to mediate the effects of stress on memory and the hippocampus, including serotonin14 and brain-derived neurotrophic factor (BDNF).15,16 Stress also results in deficits in new learning that are secondary to damage to the hippocampus.17,18 Exciting recent research has shown that the hippocampus has the capacity to regenerate neurons and that stress inhibits neurogenesis in the hippocampus.19
Studies in animals showing glucocorticoid-mediated hippocampal toxicity and memory dysfunction with stress raised the question: Does early stress, such as childhood abuse, result in similar deficits in human subjects? With this in mind, we used neuropsychological testing to measure declarative memory function in PTSD. We selected measures that were validated in studies of patients with epilepsy to be specific probes of hippocampal function. These neuropsychological measures (including delayed paragraph recall and word list learning) were correlated with a loss of neurons in the hippocampus in patients who underwent surgical resection of the hippocampus for the treatment of epilepsy.20 We initially found verbal declarative memory deficits using similar measures in Vietnam combat veterans with PTSD.21
In the first report to use brain imaging in PTSD, combat veterans were found to have an 8% reduction in right hippocampal volume, measured with magnetic resonance imaging (MRI), with no difference in comparison regions including caudate, amygdala and temporal lobe (Figure 1).
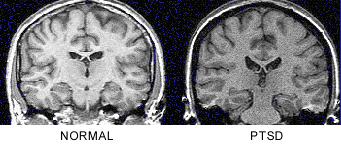
Decreases in right hippocampal volume in the PTSD patients were associated with deficits in short-term memory (r=0.64; p<0.05).22 This initial report was replicated in survivors of childhood physical and/or sexual abuse with PTSD. We found similar deficits in short-term memory as in the combat veterans, which were correlated with level of abuse as quantitated with the Early Trauma Inventory23 (Figure 2) and a 12% reduction in left hippocampal volume.24
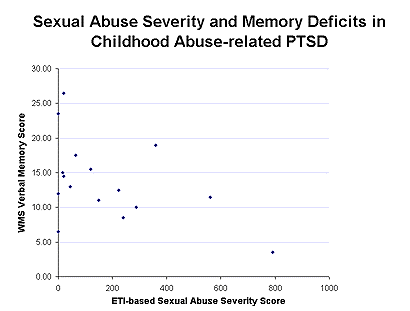
Two other replications of the original study have now been reported showing hippocampal volume reduction in PTSD.25,26 Our preliminary data show a failure of hippocampal activation measured with PET during memory retrieval in PTSD. We also showed that hippocampal volume reduction is specific to PTSD and not other anxiety disorders (panic disorder).
Studies are ongoing to assess the effects of treatments on hippocampal volume in PTSD using medications, including fluoxetine and phenytoin, that were shown in animal studies to increase dendritic branching or block the effects of stress on the hippocampus. We are also comparing monozygotic twins with and without PTSD to rule out the possibility that smaller hippocampal volume at birth can explain findings of hippocampal volume reduction in PTSD, acting as a type of risk factor.
The hippocampus has an inhibitory effect release of corticotropin releasing factor (CRF) from the pituitary. CRF plays a critical role in the stress response, both mediating peripheral HPA axis activation in stress and acting centrally to mediate fear-related behaviors and chronic stress in animals was shown to lead to chronic increases in CRF release. Again consistent with hippocampal dysfunction, we found elevations of concentrations of CRF in the cerebrospinal fluid (CSF) in PTSD.27
Empirical studies on memory and the hippocampus may shed some light on the controversy surrounding delayed recall of memories of childhood abuse. The hippocampus plays an important role in integrating or binding together different aspects of a memory at the time of recollection and is felt to be responsible for locating the memory of an event in time, place and context. We have hypothesized that atrophy and dysfunction of the hippocampus, following exposure to childhood abuse, may lead to distortion and fragmentation of memories.7 For instance, in an abused patient who was locked in the closet, there is a memory of the smell of old clothes but no visual memory of being in the closet and no affective memory of the feeling of fear. Perhaps, with psychotherapy, there is a facilitation of associations to related events that may bring all of the aspects of the memory together. Or, if the patient has an event such as being trapped in a dark elevator, the feeling of fear with darkness and the enclosed space may be enough to trigger a recollection of the entire memory.
The effects of childhood trauma on memory and the brain also have important implications for public health policy. This is especially pertinent for inner-city children who often witness violent crimes in their neighborhoods and families, in addition to trauma, such as childhood abuse. If abused children have damage to brain areas involved in learning and memory, this may put them at a serious disadvantage that programs such as Head Start will not be able to overcome. Consistent with this, traumatized Beirut adolescents with PTSD had deficits in academic achievement, compared to non-traumatized adolescents and traumatized adolescents without PTSD.28
Dysfunction of the medial prefrontal cortex in PTSD
Abnormalities of other brain areas (Figure 3), including medial prefrontal cortex, are also associated with PTSD.
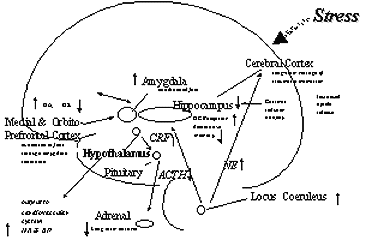
Medial prefrontal cortical areas modulate emotional responsiveness and mediate conditioned fear responses to fear-inducing stimuli through inhibition of amygdala function.29 We have hypothesized that dysfunction in these regions may underlie pathological emotional responses in patients with PTSD.30 This area also has important functional connections with the hippocampus. In several studies using positron emission tomography (PET) imaging of brain function in PTSD, we have found dysfunction of the medial prefrontal cortex and hippocampus during provocation of PTSD symptoms and presentation of traumatic cues. We stimulated PTSD symptoms with the noradrenergic agent, yohimbine. We found a relative failure of activation in metabolism in parts of medial prefrontal cortex (orbitofrontal), decreased function in hippocampus, in comparison to placebo and in comparison to responses in healthy subjects.31
In a study using combat-related slides and sounds to provoke PTSD symptoms, combat veterans with PTSD (but not combat veterans without PTSD) demonstrated a decrease in blood flow in the medial prefrontal cortex (Brodmann’s area 25 or subcallosal gyrus) (Figure 4), with a failure of activation in anterior cingulate (area 32 and 24) and increased activation in posterior cingulate, motor cortex and lingual gyrus.32
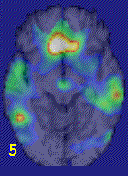
Women with childhood sexual abuse and PTSD, compared to sexually abused women without PTSD, exposed to personalized scripts of childhood sexual abuse, again showed decreased blood flow in medial prefrontal cortex (area 25) and failure of activation in anterior cingulate, with increased blood flow in posterior cingulate and motor cortex. PTSD women also had decreased blood flow in right hippocampus. These imaging findings are consistent with dysfunction of medial prefrontal cortex and hippocampus in PTSD that may underlie pathological emotions in these patients.
Summary
This review has covered the broad impact of childhood abuse and other traumas on memory and the hippocampus. The hippocampus, a brain area involved in verbal declarative memory, is particularly sensitive to stress. Patients with combat and abuse-related PTSD were shown to have smaller hippocampal volume and deficits in hippocampal-based verbal declarative memory functions. Hippocampal dysfunction may underlie many symptoms of PTSD and may explain elevations in concentrations of CRF in PTSD. Another brain area affected by stress is the medial prefrontal cortex. Functional imaging studies show dysfunction in this area (as well as hippocampus) during provocation of PTSD symptoms and presentation of traumatic reminders. Medial prefrontal cortical inhibition of amygdala responsiveness is felt to underlie extinction to fear responding. Dysfunction of this area in abuse-related PTSD may lead to problems modulating emotion in PTSD.

References
1. McCauley J, Kern DE, Kolodner K, Dill L, Schroeder AF, DeChant HK, Ryden J, Derogatis LR, Bass EG (1997). Clinical characteristics of women with a history of childhood abuse: Unhealed wounds. JAMA 277:1362-1368. return
2. Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB (1995). Posttraumatic stress disorder in the national comorbidity survey. Arch Gen Psychiatry 52:1048-1060. return
3. Bremner JD, Marmar C (eds.) (1998): Trauma, Memory and Dissociation, APA Press, Washington DC. return
4. Saigh PA, Bremner JD (Eds.) (1999). Posttraumatic Stress Disorder: A Comprehensive Text, Allyn & Bacon, New York. return
5. Bremner JD, Narayan M (1998): The effects of stress on memory and the hippocampus throughout the life cycle: Implications for childhood development and aging. Develop Psychopath 10:871-886. return
6. Bremner JD, Southwick SM, Charney DS (1999): The neurobiology of posttraumatic stress disorder: An integration of animal and human research. In: Saigh, P., Bremner, J.D. (Eds.): Posttraumatic Stress Disorder: A Comprehensive Text, Allyn & Bacon, New York, pp. 103-143. return
7. Bremner JD, Krystal JH, Charney DS, Southwick SM (1996): Neural mechanisms in dissociative amnesia for childhood abuse: Relevance to the current controversy surrounding the “False Memory Syndrome”. Am J Psychiatry 153(7):FS71-82. return
8. McEwen BS, Angulo J, Cameron H, Chao HM, Daniels D, Gannon MN, Gould E, Mendelson S, Sakai R, Spencer R, Woolley C (1992): Paradoxical effects of adrenal steroids on the brain: Protection versus degeneration. Biol Psychiatry 31:177-199. return
9. Sapolsky RM (1996). Why stress is bad for your brain. Science 273:749-750. return
10. Uno H, Tarara R, Else JG, Suleman MA, Sapolsky RM (1989): Hippocampal damage associated with prolonged and fatal stress in primates. J Neurosci 9:1705-1711. return
11. Sapolsky RM, Uno H, Rebert CS, Finch CE (1990): Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci 10:2897-2902. return
12. Woolley CS, Gould E, McEwen BS: Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res 1990; 531:225-231. return
13. Virgin CE, Taryn PTH, Packan DR, Tombaugh GC, Yang SH, Horner HC, Sapolsky RM (1991). Glucocorticoids inhibit glucose transport and glutamate uptake in hippocampal astrocytes: implications for glucocorticoid neurotoxicity. J Neurochem 57:1422-1428. return
14. McEwen BS, Conrad CD, Kuroda Y, Frankfurt M, Magarinos AM, McKittrick C (1997). Prevention of stress-induced morphological and cognitive consequences. Eur Neuropsychopharm 7:(suppl)3:S322-328. return
15. Smith MA, Makino S, Kvetnansky R, Post RM (1995). Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNA in the hippocampus. J Neurosci 15:1768-1777. return
16. Nibuya M, Morinobu S, Duman RS (1995). Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci 15:7539-7547. return
17. Luine V, Villages M, Martinex C, McEwen BS (1994): Repeated stress causes reversible impairments of spatial memory performance. Brain Res 639:167-170. return
18. Bodnoff SR, Humphreys AG, Lehman JC, Diamond DM, Rose GM, Meaney MJ (1995): Enduring effects of chronic corticosterone treatment on spatial learning, synaptic plasticity, and hippocampal neuropathology in young and mid-aged rats. J Neurosci 15:61-69. return
19. Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E (1998) Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. PNAS 95:3168-3171. return
20. Sass KJ, Spencer DD, Kim JH, Westerveld M, Novelly RA, Lencz T (1990). Verbal memory impairment correlates with hippocampal pyramidal cell density. Neurology 40:1694-1697. return
21. Bremner JD, Scott TM, Delaney RC, Southwick SM, Mason JW, Johnson DR, Innis RB, McCarthy G, Charney DS (1993): Deficits in short-term memory in post-traumatic stress disorder. Am J Psychiatry 150:1015-1019. return
22. Bremner JD, Randall PR, Scott TM, Bronen RA, Delaney RC, Seibyl JP, Southwick SM, McCarthy G, Charney DS, Innis RB (1995): MRI-based measurement of hippocampal volume in posttraumatic stress disorder. Am J Psychiatry 152:973-981. return
23. Bremner JD, Randall PR, Capelli S, Scott T, McCarthy G, Charney DS (1995): Deficits in short-term memory in adult survivors of childhood abuse. Psych Res 59:97-107. return
24. Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure CM, Capelli S, McCarthy G, Innis RB, Charney DS (1997): MRI-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse: A preliminary report. Biol Psychiatry 41:23-32. return
25. Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B (1997): Hippocampal volume in women victimized by childhood sexual abuse. Psychol Medicine 27:951-959. return
26. Gurvits TG, Shenton MR, Hokama H, Ohta H, Lasko NB, Gilberson MW, Orr SP, Kikinis R, Lolesz FA, McCarley RW, Pitman RK (1996): Magnetic resonance imaging study of hippocampal volume in chronic combat-related posttraumatic stress disorder. Biol Psychiatry 40:192-199. return
27. Bremner JD, Licinio J, Darnell A, Krystal JH, Owens M, Southwick SM, Nemeroff CB, Charney DS (1997): Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry 154:624-629. return
28. Saigh PA, Mroweh M, Bremner JD (1997) Scholastic impairments among traumatized adolescents. Beh Res Ther 35:429-436. return
29. Morgan MA, LeDoux JE (1995): Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci 109:681-688. return
30. Bremner JD, Krystal JH, Southwick SM, Charney DS (1995) Functional neuroanatomical correlates of the effects of stress on memory. J Trauma Stress 8:527-554. return
31. Bremner JD, Innis RB, Ng CK, Staib L, Duncan J, Bronen R, Zubal G, Rich D, Krystal JH, Dey H, Soufer R, Charney DS (1997): PET measurement of central metabolic correlates of yohimbine administration in posttraumatic stress disorder. Arch Gen Psychiatry 54:246-256. return
32. Bremner JD, Staib L, Kaloupek D, Southwick SM, Soufer R, Charney DS (1999): Positron emission tomographic (PET)-based measurement of cerebral blood flow correlates of traumatic reminders in Vietnam combat veterans with and without posttraumatic stress disorder. Biol Psychiatry (In press). return
created 3/3/99, reviewed 3/5/00; modified 3/7/99; end-date 3/7/02.
